Policy Interpretation: Import & Export of Medical Devices
Medical Devices refer to instruments, equipment, appliances, IVD Reagents, reference substances, material and other similar or related articles, including necessary computer software, which are used directly or indirectly on human body. Their functions and usages are mainly obtain through physical way instead of pharmacological, immunological or metabolic ways(or the three ways take part in but only play a supporting role ). and the purposes of medical devices are as follow,
1.To diagnose, prevent, monitor, treat or alleviate diseases.
2.To diagnose, , monitor, treat,alleviate or diseases,compensate functionally injuries.
3.To test, replace, adjust or support physical structure or process.
4.To support or sustain life
5.To control pregnancy
6.To provide information for medical treatment or Diagnostic purpose by examine samples from human body.
Classification
1.China implements classification management on medical devices based on degrees of risk.
Category Onerefers to medical devices whose safety and effectiveness can be guaranteed through routine management. For example, surgical instruments like knifes,scissors, pincers, tweezers and hooks, scraping plates, medical X-ray films, surgical gowns, surgical hats, examination gloves, gauze bandages, drainage packs,etc.
Category Two refers to medical devices whose safety and effectiveness shall be controlled. For example, medical suture needles, blood pressure needles, clinical thermometers, electrocardiographs, electroencephalographs, microscopes, acupuncture needles, bio-chemical analysis system, hearing-aids, ultrasonic disinfection equipment, non-absorbable suture strings, condoms, etc.
Category Three refers to medical devices whose safety and effectiveness must be strictly control as medical devices in this category have potential dangers for human body. For example, implantable cardiac pacemaker, corneal contact lens, intraocular lens, super sparing tumor focusing knifes, hemodialysis devices, implant devices, intravascular stents, comprehensive anaesthesia machines, dentistry implant materials, medical absorbable suture strings, Endovascular catheters, etc.
Reminder
Assessment of risk levels of medical devices shall consider intended purposes, structure characteristics, using methods, etc.
2 Classification Catalogue of Medical Devices
Former China Food & Drug Administration revised Classification Catalogue of Medical Devices and released it officially on September,2017. The revised Classification Catalogue of Medical Devices, enforced officially on Aug 1, 2018, has 22 sub-catalogs, and refines and adjusts 260 product categories to 206 Category One and 1157 Category Two, and forms three-layer catalogue structure.
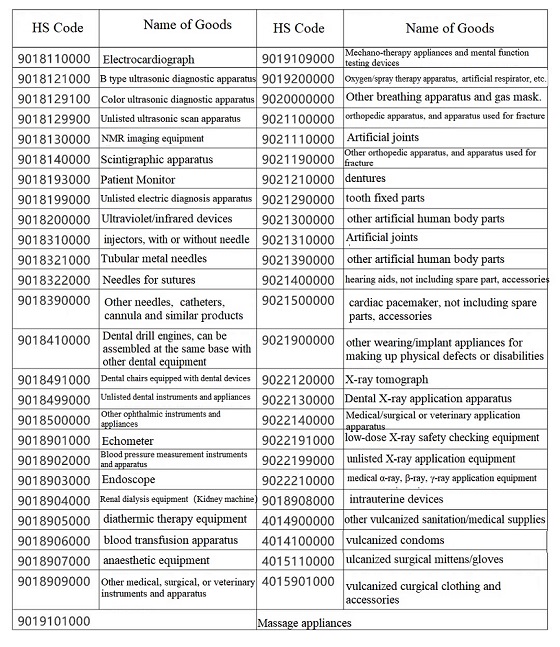
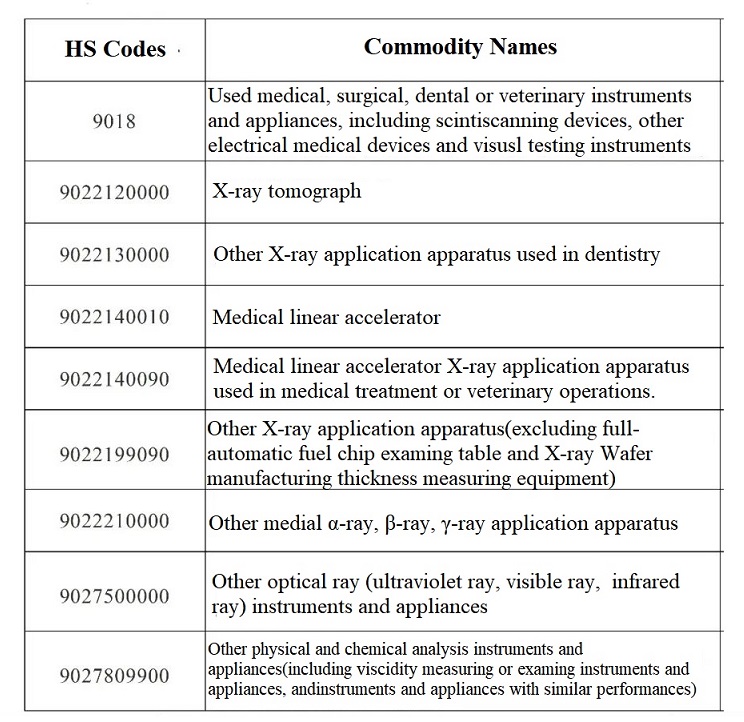
3.What are the HS codes for Medical Devices?
HS codes for medical devices are mainly in HS 9018¡¢9021¡¢9022¡¢9402. When making import & export declarations or export rebates, one shall search and use matching HS codes based on different products. As below are part of HS codes and names for medical devices for reference.

Filing and Registration
Product filing management is implemented on Class One medical devices, while product registration management is executed on Class Two & Three medical devices. One overseas filing/registration applicant that plans to export medical devices to our country shall , through his appointed legal bodies of enterprise in our country, submit filing/registration materials and evidentiary material(s) that prove the medical devices are allowed to be sold on the market by the competent department of the country/region where the filing/registration applicant is from. Applicants of innovative medical devices that have not yet been sold on the overseas market do not have to submit evidentiary material(s) that prove the medical devices are allowed to be sold on the market by the competent department of the country/region where the filing/registration applicants are from.
Import and Export
1 Export of Medical Devices
Enterprises that export medical devices shall guarantee that exported medical devices meet requirements of importing countries/regions. Normally, commodity inspection is not required.
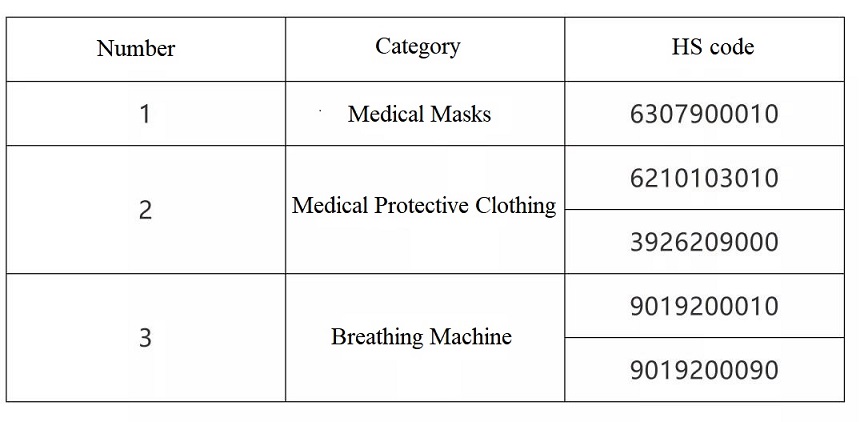
What should be noted is that, pursuant to 2020 #53 & #124 Announcements of General Administration of Customs, export commodity inspection shall be implemented on medical devices under below five HS codes.

Reminder
Medical devices under HS codes 3005901000 and 3005909000 that are dangerous goods, and medical devices under HS code 3808940010 that are dangerous chemicals shall be executed in accordance with the inspection supervision requirements for exported dangerous goods or exported dangerous chemicals.
2 Import of Medical Devices
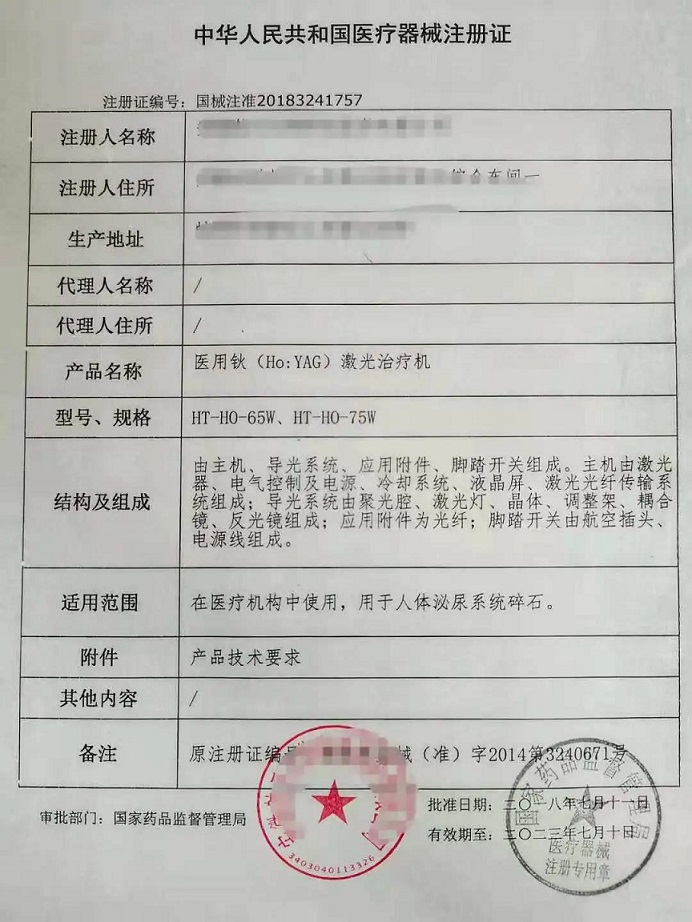
¡öImported medical devices shall be medical devices that have been registered or filed. Registration Certificate for Medical Devices is valid for five years. Extension application shall be made to the original registration department 6 months before validity expires if needed. As below is an example of Registration Certificate for Medical Devices.

¡öRegistered Medical devices refer to medical devices whose registration certificate is consistent with the content of its appendix and that are produced within the validity of their registration certificate.
¡öMedical institutions can import a small quantity of Category Two and Category Three medical devices that are for urgent clinical needs after approved by Drug Administration Department of State Council or by provincial or municipal people¡¯s governments that are authorized by State Council. The imported medical devices of this type shall be used for specific medical purposes at appointed medical institutions.
¡öChina customs carries out online verification on electronic data of filing/registration certificate for imported medical devices and electronic data of declaration form for imported medical devices.
¡ö When declare imported medical devices, contract, invoice, packing list and filing/registration certificate for imported medical devices shall be provided.
¡öImported medical devices shall come with Chinese user¡¯s manual and Chinese label(below pictured are examples) that meets relevant regulation and compulsory requirements. The Chinese user¡¯s manual shall specify country of origin, and name, address, contact info of our country¡¯s enterprise legal person appointed by overseas medical medical devices registrant/filing person.


¡öChina customs carries inspection on imported medical devices. Those inspected as unqualified are prohibited from importing. Customs will check if the info of the commodities being inspected, declared info and admittance info are consistent. Customs will also check if the name plate info is accordant with the manufacturer, name of commodity, model, specifications as registered or filed.


Special Requirements
Imported Heart Pacemakers
Customs appointed by General Administration carry out inspection supervision on imported heart pacemaker and appoint medical devices testing organizations approved by the state to test imported heat pacemaker. Beijing customs, Shanghai customs and Haikou customs are appointed inspection enforcement bodies for imported heart pacemaker.


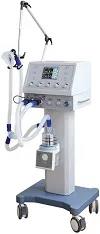
Imported Breathing Machines
Customs enforce key supervisions on high imported breathing machines. The inspection supervision jobs are carried out by customs in Beijing, Tianjin, Dalian, Shanghai, Qingdao, Wuhan and Guangzhou. We are Customs Broker in Shanghai and Customs Agent in Guangzhou for imported medical devices. Welcome to send us inquiries.

Imported Donated Medical Devices
It is prohibited that overseas donors carry secretly articles listed in Goods Catalog Banned from Importing or articles that are harmful to our country¡¯s environment, public sanitation, social morality and politics. The medical devices donated to our country. The donated medical devices shall be new and have been filed or registered in China
Medical Devices that are banned from Importing
Used medical devices that are expired, ineffective and obsolete are prohibited from importing into our country.
The Catalog for Used Mechanical and Electrical Products that are Banned from Import was adjusted in accordance with 2018 #108 announcement of General Administration of Customs and has been executed from Jan 1, 2019. As below is the list covering Medical Devices that are banned from Importing.

Related Reading£º


